The Spanish standardization body has
updated their specifications for face coverings. New versions of specifications for hygienic masks have been released in September 2021. The updated versions below aim to provide a better alignment with the CSM/115/2021 guidance with regards to marking and instructions for use:
UNE 0064-1:2021 Non-reusable hygienic masks Part 1: For Adults use
UNE 0064-2:2021 Non-reusable hygienic masks Part 2: For Children use
UNE 0065:2021 Reusable hygienic masks for Adult and Children
Hygienic masks must comply with the following basic principles:
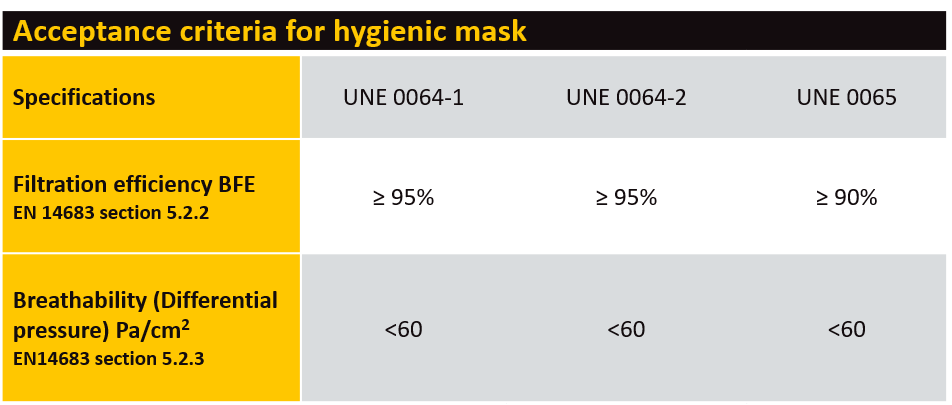
There are no major changes to performance requirements such as filtration or breathability (see table). The key changes allow manufacturers to create changes to design, shape or sizes of masks, provided they still need to comply with all a criterion stated within the specifications.
Tests for hygienic masks should be performed by ISO/ IEC 17025 accredited laboratories or accredited by accreditation body that is a signatory to the mutual recognition agreement of International Laboratory Accreditation Cooperation (ILAC).